Abstract
Background Many guidelines and expert opinions do not recommend inherited thrombophilia testing in unselected patients with venous thromboembolism (VTE), and per American Society of Hematology Choosing Wisely Recommendations, inherited thrombophilia testing is not recommended in patients with venous thromboembolism occurring in the setting of major transient risk factors. Furthermore, non-hematologists may lack knowledge on the effect of direct oral anticoagulants on these assays. From 2019 to 2021, 75% of inherited thrombophilia testing (protein C, S and antithrombin deficiencies and Factor V Leiden and prothrombin mutations) at VA Connecticut were ordered by non-hematologists. Of these orders, 55% of tests were ordered by primary care clinicians, 29% by inpatient teams, and the remainder by non-hematology specialists. Approximately 10% of testing by non-hematologists had abnormal results, generating further hematology consults and workup. Given that testing has limited clinical utility and can result in unnecessary downstream healthcare use, there is a need to improve guideline concordant and appropriate thrombophilia testing by non-hematologists.
Methods The aim of this multidisciplinary quality improvement project was to decrease the number of inherited thrombophilia tests ordered by non-hematologists at VA Connecticut by 25% six months after our intervention implementation. Quality Improvement (QI) principles were used to identify root causes, which included complexity of and confusion over appropriate patient selection for testing, a lack of awareness of potential false positives with active anticoagulant use, limitations of our electronic medical record system (EMR) to provide clinical decision support tools to triage testing or provide education, and more. After multidisciplinary stakeholder engagement and feedback from hematology, internal medicine, laboratory medicine, and clinical informatics, we established a best practice alert (BPA) for inpatient and outpatient inherited thrombophilia testing at the time of order entry. Our BPA notified the ordering clinician that inherited thrombophilia testing rarely impacts the choice or duration of anticoagulation and is not recommended for most patients. The BPA recommended that as an alternative, clinicians can consider a hematology electronic consult (e-consult) for guidance and education regarding appropriate patient selection for testing. Data was collected from our EMR seven months after intervention initiation and analyzed via Excel 365. A control chart was used to assess the outcome measure, which was the number of orders for inherited thrombophilia testing by non-hematology clinicians per month. Our balancing measure was the number of hematology e-consults for inherited thrombophilia per month. Institutional pricing was used to determine costs associated with testing and potential cost savings.
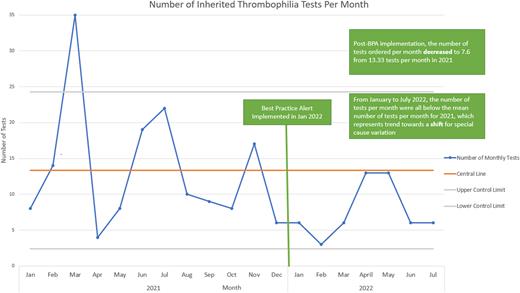
Results After our BPA implementation in January 2022, the mean number of monthly inherited thrombophilia tests from January to July 2022 decreased to 7.6 tests from 13.3 tests in 2021. All 7 monthly test order numbers post-implementation were below the mean, consistent with a potential trend towards a special cause variation shift. Inpatient testing decreased from 40 total tests in 2021 to 7 tests since implementation. The average cost of testing per month decreased from $653 to $366 with an estimated $2004 saved after intervention implementation. The mean number of hematology e-consults for inherited thrombophilia related questions increased to 3.4 consults per month during the first five months of the intervention period from 2.5 consults per month in 2021.
Conclusion A best practice alert in the EMR reminding clinicians about the limited role of inherited thrombophilia testing in unselected patients and the option for hematology e-consult for further specialist guidance was associated with a trend towards reduced inherited thrombophilia testing. Furthermore, there were decreased costs associated with testing without any substantial increase in workload for the hematology service. The BPA remains in place, and data collection and analysis are ongoing.
Disclosures
Hauser:Transfusion Alloantibody Exchange: Other: Founder. Rose:BMS: Other: VA Connecticut Research & Education Foundation to support patients enrolled onto their sponsored studies; Janssen: Other: VA Connecticut Research & Education Foundation to support patients enrolled onto their sponsored studies.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal